B. Complete the online Data Access Request (DAR) form
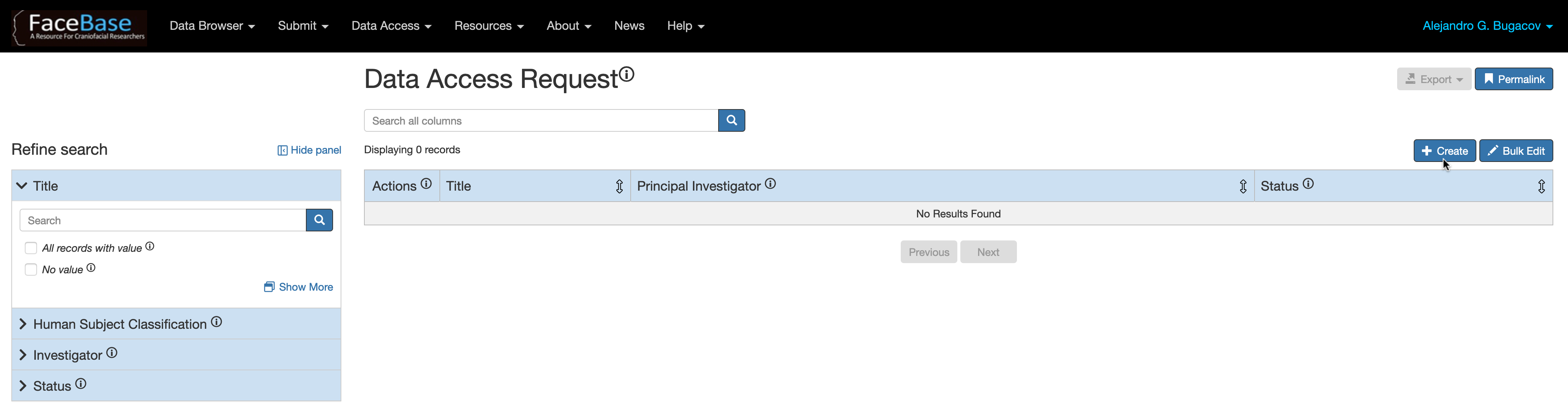
Create new DAR request
Go to the Data Access Request (DAR) page, log in and click the + Create button at the top right corner of the page (The DAR form will open in a new tab).
You must first login with your FaceBase User account in order to edit or start a new DAR form. If you are not registered as a FaceBase user, click on the Sign Up link at the top-right corner of the page and follow the instructions to log in through Globus and join the “FaceBase Users” group. You will receive email notification once your request is approved by the FaceBase Hub.
Complete fields (1) to (14) on the DAR form
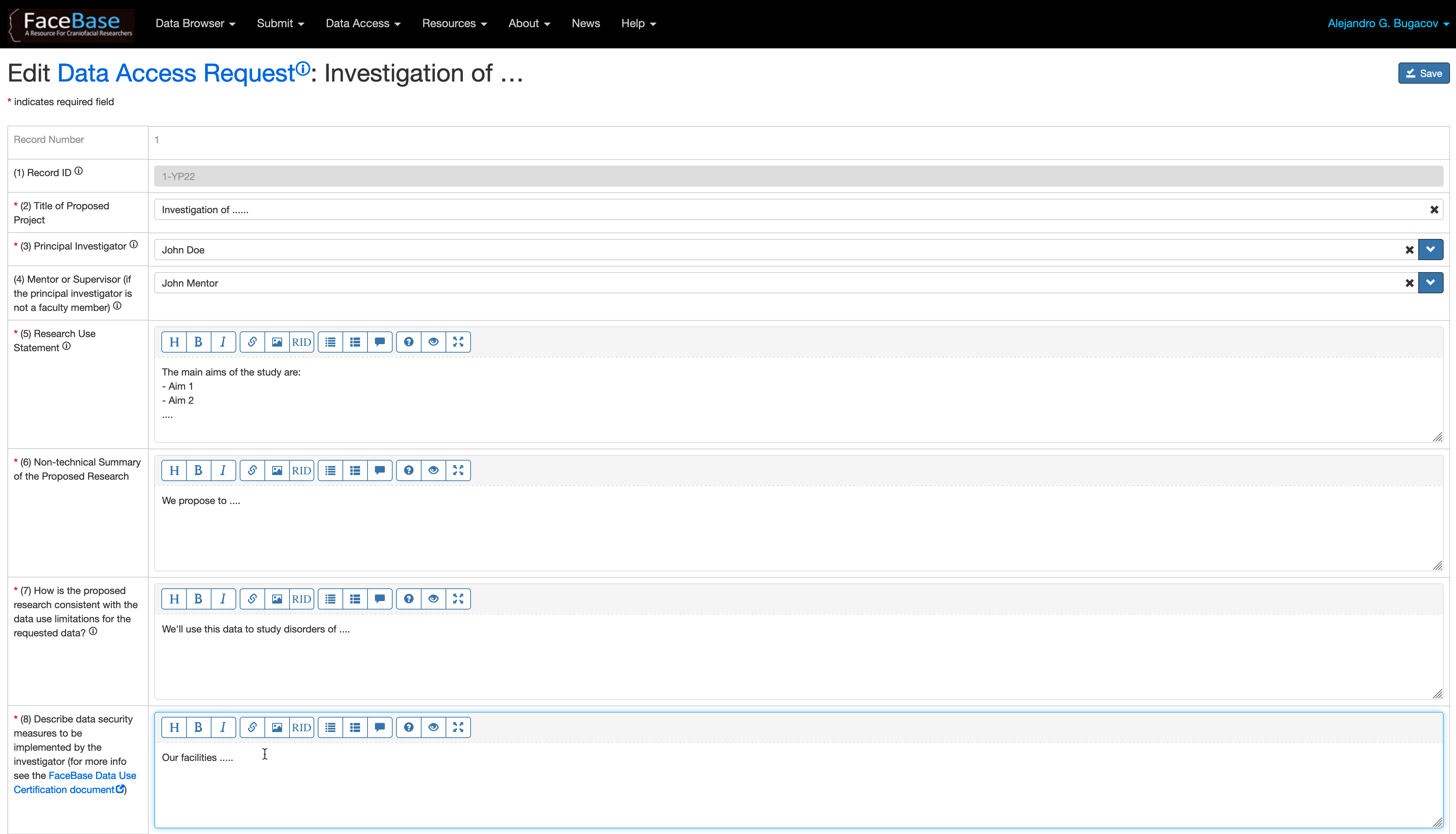


- A red asterisk denotes the field is required to create the request. Other fields can initially be left blank and completed at a later time.
-
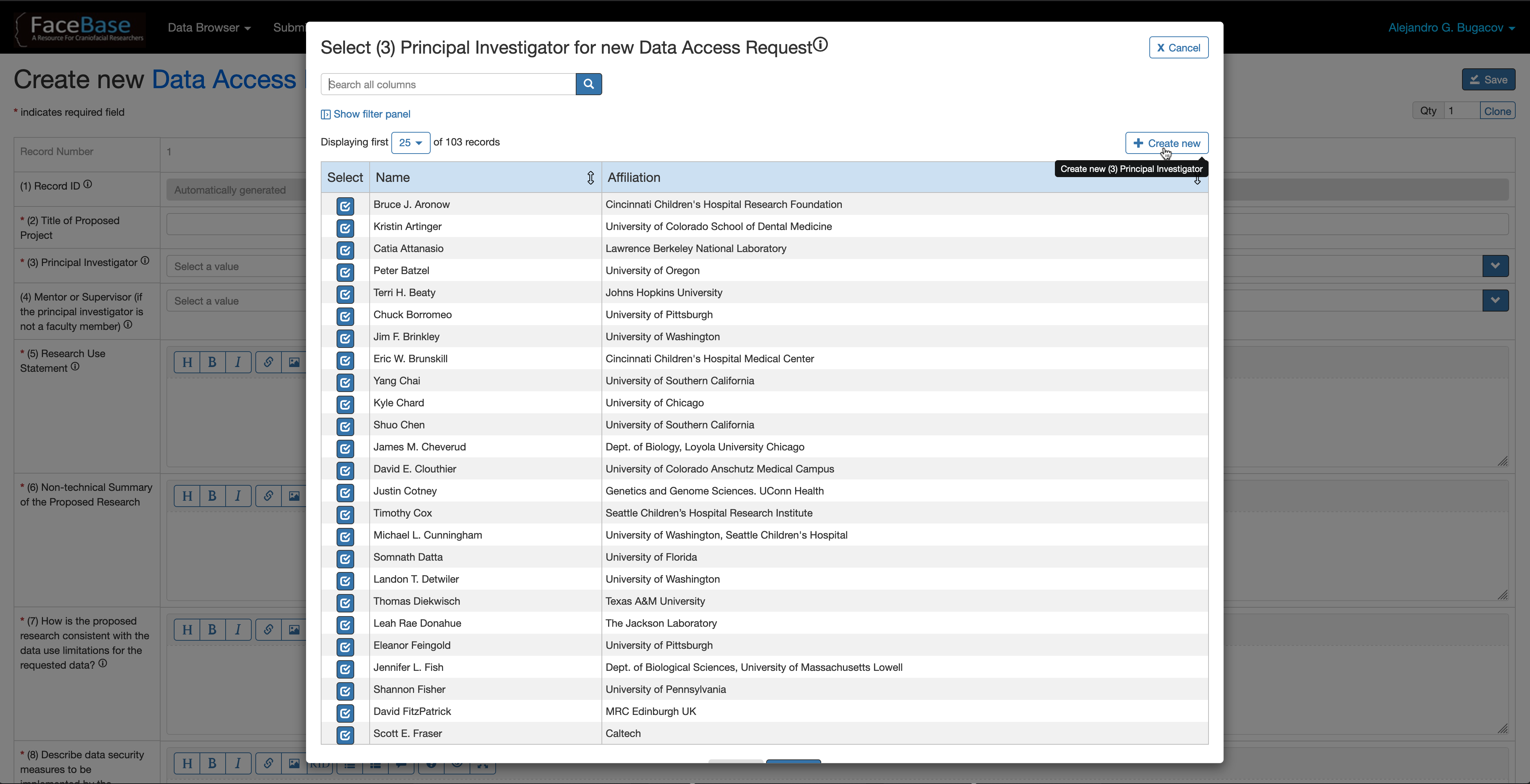
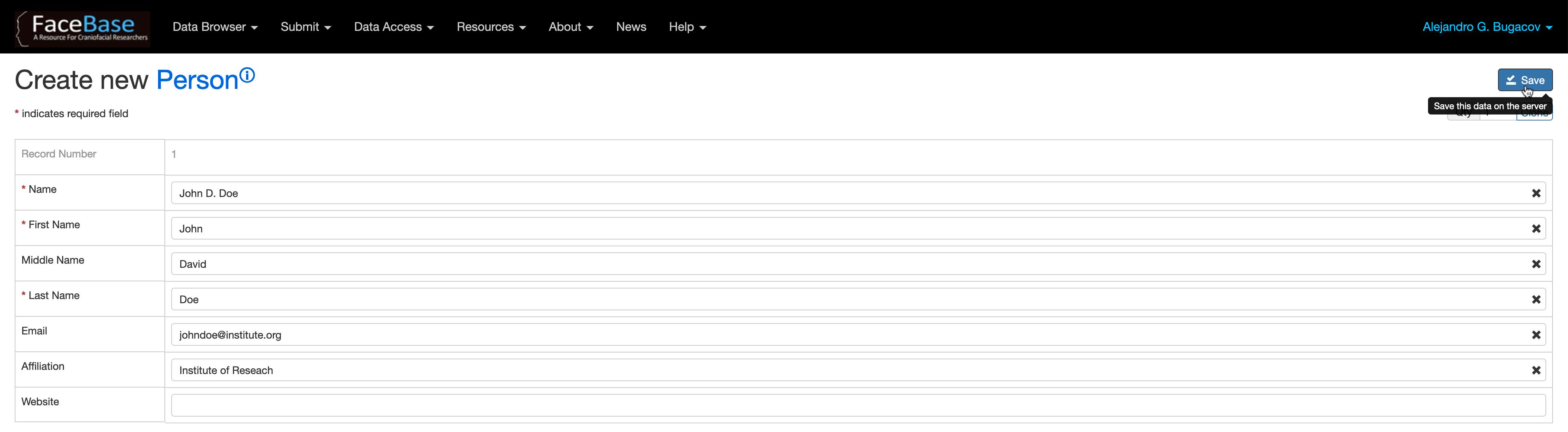
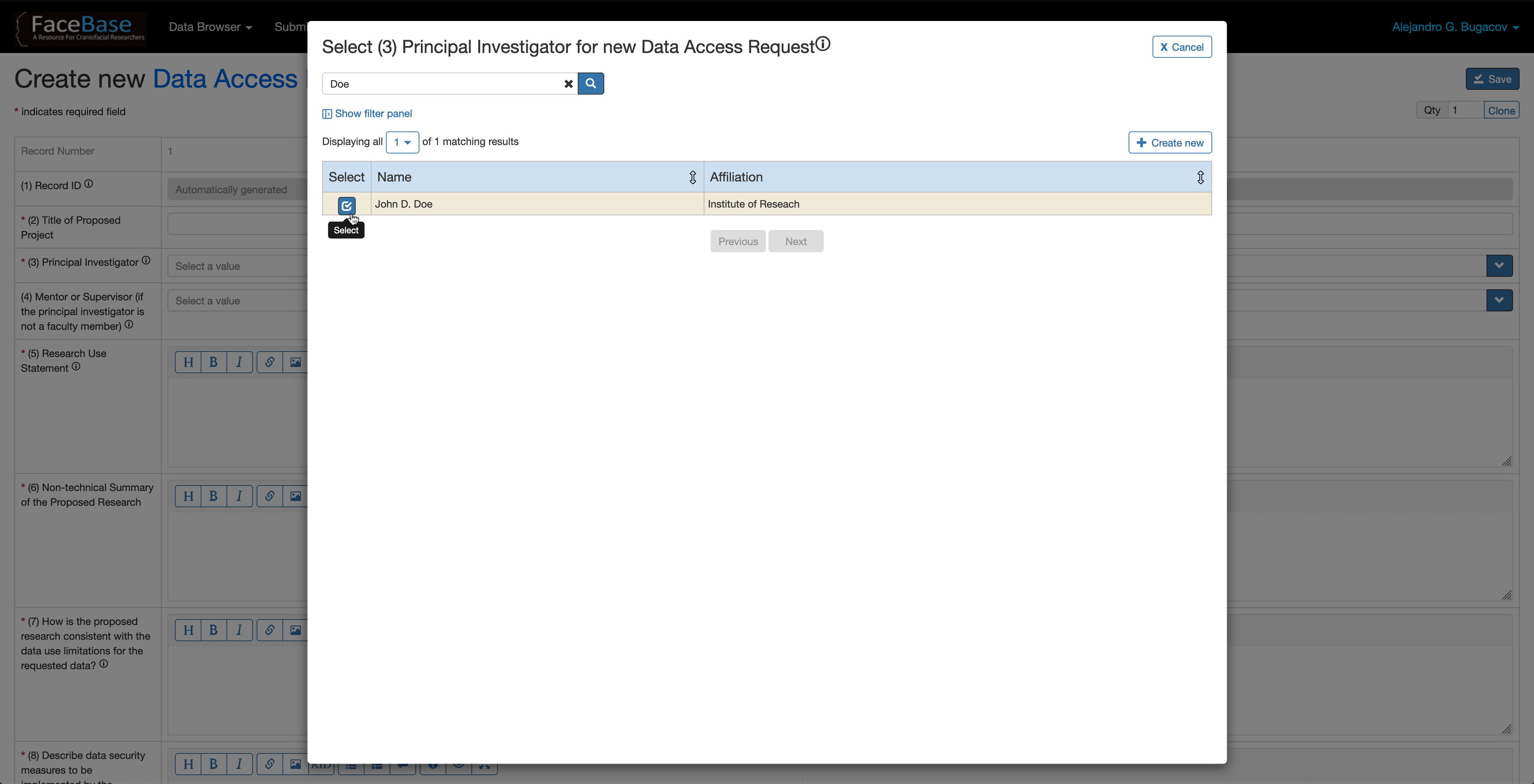
In fields (3) Principal Investigator and (4) Mentor or Supervisor, if you don’t find the PI or Mentor names listed there, click on the + Create New button to create a new person record in the system. Then select the newly created record from the list of investigators.
- If the dataset requires IRB approval (per the Data Use Limitations field), then in fields (12) Submit a copy of the IRB letter… and (13) Submit a copy of the IRB-approved protocol… click on the Select file button to the right of each field and select the file to upload from your local file system. The files will be uploaded to the FaceBase site once you save the form.
- Fields (14) and (15) should initially be left blank. Once you print the complete DUC and DAR forms and obtain all required signatures you must edit this form to add the signed documents to these fields.
Save the form
- Make sure the Status field remains “In progress (Pending Signature)”
- Click the Save button in the upper right corner to save all information entered in the form at this point.
If you are a researcher at an academic institute outside the US or if you are unfamiliar with IRBs…
Please see our relevant FAQ on the main FaceBase site that points you to resources at the US NIH website for more information on IRBs. For institutes outside the US, they usually have a comparable “ethics board” or something similar that can provide the necessary information. You may also contact your own university offices that deal with research on human subjects and they should provide guidance.